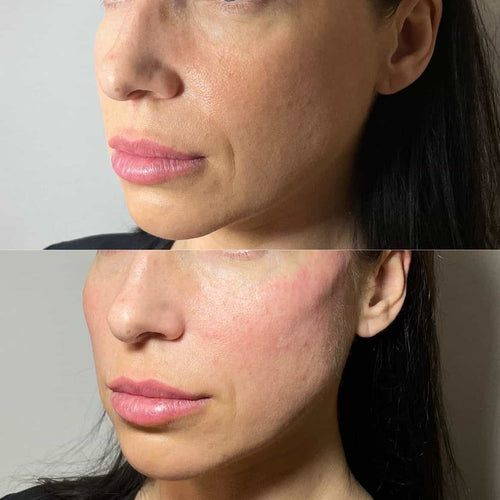
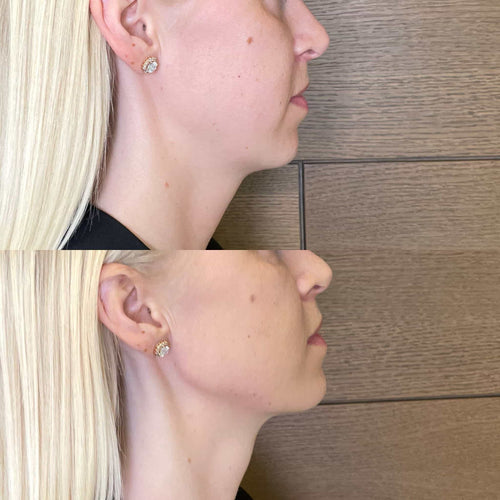
Schedule Your Dermal Filler Appointment at It’s Me and You Clinic with Dr. Laura Geige
Understanding the Science Behind Baby Botox
The term “Baby Botox” refers to a smaller dose of _Botulinum Toxin Type A_, commonly used in cosmetic treatments, particularly for wrinkles and facial folds. This variation has gained popularity due to its efficacy and the associated benefits of reduced side effects.
Unlike traditional Botox treatments that require higher doses of _Botulinum Toxin_, Baby Botox employs a lower concentration, typically between 2-5 _Units_ of _Botulinum Toxin Type A_ per area. This difference in dose has significant implications on the treatment’s outcome and patient experience.
One key aspect that sets Baby Botox apart is its application as an off-label treatment for more than just facial wrinkles. It can be used to treat various conditions, such as excessive sweating (hyperhidrosis) and migraines, by employing a broader range of _Botulinum Toxin_ dosages.
The lower dose used in Baby Botox is also beneficial because it results in less pronounced side effects, such as bruising, swelling, or eyelid drooping. Patients often report fewer complications when undergoing this treatment compared to traditional Botox.
Another critical difference between Baby Botox and traditional Botox lies in the treatment’s duration of effect. Due to its lower potency, Baby Botox may require more frequent injections, which can occur every 4-6 weeks. This schedule helps maintain optimal results without overusing or underusing _Botulinum Toxin_.
Furthermore, the use of smaller doses in Baby Botox has sparked research into potential health benefits. Some studies suggest that this variation could be employed for treating conditions such as chronic pain management or even certain types of seizures.
The lower dose and broader application capabilities of Baby Botox have contributed to its rising popularity in medical spas and dermatological practices worldwide. As a result, more patients are seeking this innovative treatment option to address their cosmetic and functional concerns.
Baby botox is a lesser-known version of the popular neurotoxin treatment, often used to address facial wrinkles and fine lines.
The name “baby botox” refers to the smaller dose of _Botox_ used, typically between 10-20 units, which is significantly less than the standard dosage of 50-100 units commonly administered for traditional Botox treatments.
Get Your Dermal Filler Consultation with Dr. Laura Geige at It’s Me and You Clinic
This reduced dosing makes baby botox an ideal solution for individuals with mild facial wrinkles or those who are new to Botox treatments and want to gauge their reaction before committing to a full treatment.
One key advantage of baby botox is its ability to provide a more subtle, natural-looking effect. The smaller dose allows for a more nuanced expression of the facial muscles, resulting in a softer, more relaxed appearance.
Another benefit of baby botox is its lower cost compared to traditional Botox treatments. Since less _Botox_ is required, the price per unit is significantly reduced, making it a more accessible option for those seeking a wrinkle-reducing treatment.
From a scientific perspective, the difference between baby botox and traditional botox lies in the way they interact with the facial muscles. Traditional Botox works by temporarily blocking the release of _acetylcholine_, a neurotransmitter responsible for muscle contraction. This blockade leads to a reduction in muscle activity, resulting in smoother, more relaxed skin.
Baby botox, on the other hand, uses a smaller dose of Botox that is specifically designed to target the smaller facial muscles involved in expression and relaxation. By targeting these specific areas, baby botox can provide a more precise and targeted effect, minimizing the risk of over-treatment or unintended side effects.
From an _anatomical_ standpoint, the difference between baby botox and traditional botox is also significant. Traditional Botox treatments often target the crow’s feet area (periorbital region), forehead lines, and frown lines (glabellar region). Baby botox, however, may focus more on the nasolabial folds (the wrinkles that form between the nose and mouth) or the marionette lines (the wrinkles that form around the mouth).
Regardless of its smaller dosage, baby botox remains a highly effective treatment for addressing facial wrinkles and fine lines. By providing a subtle yet noticeable effect, baby botox can be an excellent option for those seeking a more understated approach to anti-aging.
The concept of “Baby Botox” has gained popularity in recent years, particularly among parents and medical professionals alike, who are interested in harnessing the therapeutic benefits of Botox for infants. However, what sets Baby Botox apart from traditional Botox is not just the dosage, but also the underlying science behind its application.
In 2009, Dr. Jean Carruthers, a renowned dermatologist and researcher, first proposed the idea of using lower doses of Botox for infantile facial spasms (IFS). This was based on the observation that infants have natural muscle tone and fat reserves that can help reduce the severity of spasms.
The human body is comprised of approximately 640 muscles, with the majority being responsible for voluntary movements. However, in infants, many of these muscles are still developing and are characterized by a unique mixture of relaxation and contraction states. This relaxed muscle state plays a crucial role in preventing excessive spasms and muscle tension.
The key to understanding Baby Botox lies in the concept of neuroplasticity, which refers to the brain’s ability to reorganize and adapt throughout life. In infants, the brain is highly malleable, allowing for rapid learning and adaptation to new stimuli. This neuroplasticity can be leveraged to treat various conditions, including IFS.
When Botox is injected into an infant’s muscles, it temporarily blocks the release of a neurotransmitter called acetylcholine, which is responsible for muscle contractions. By reducing the amount of acetylcholine released, the muscle spasms are reduced, resulting in a smoother and more relaxed facial expression.
The dosage of Baby Botox is significantly lower than that used for traditional Botox treatments. Studies have shown that a dose as low as 0.01-0.1 units per kilogram of body weight can be effective in treating IFS. This reduced dosage is thought to be due to the natural muscle tone and fat reserves of infants, which help distribute the Botox toxin more evenly throughout the muscle.
Furthermore, the use of Baby Botox has been shown to have a range of additional benefits for infants, including improved sleep patterns, reduced irritability, and enhanced overall quality of life. These benefits are thought to be due to the reduction in muscle tension and spasms, which can lead to a more relaxed and calm infant.
In contrast, traditional Botox treatments often require much higher doses, typically ranging from 10-20 units per session. This higher dosage is necessary to achieve significant reductions in facial wrinkles and muscle spasms, but it also increases the risk of side effects such as headaches, droopy eyelids, and facial asymmetry.
In conclusion, Baby Botox offers a unique therapeutic approach for treating infantile facial spasms and other conditions. By leveraging the natural muscle tone and fat reserves of infants, and utilizing lower doses of Botox, medical professionals can provide a safer and more effective treatment option for young patients.
Factors Influencing Efficacy and Safety
The efficacy and safety of cosmetic procedures, such as facial rejuvenation treatments like Botox, are influenced by a multitude of factors. One key factor is _**Muscle Type**_, with different muscle types responding differently to the same treatment.
Facial muscles can be broadly categorized into three main types: voluntary, superficial, and deep. Voluntary muscles, such as those controlling facial expressions, are typically more responsive to Botox injections. Superficial muscles, like the orbicularis oculi, which surrounds the eye, can also be treated with Botox, but may require more precise administration.
Deep muscles, on the other hand, such as those involved in the forehead’s corrugator supercilii and procerus muscles, are generally less responsive to Botox. This is because deep muscles have thicker tissue and a higher concentration of smooth muscle cells, which makes it more challenging for the toxin to spread effectively.
The depth at which Botox is administered also plays a crucial role in its efficacy and safety. **Superficial** injections, typically targeting superficial muscles like the frontalis and orbicularis oculi, are generally less invasive and have fewer complications compared to deep injections.
Deep injections, while more effective for treating deep facial muscles, carry a higher risk of side effects such as eyelid drooping, eyebrow ptosis, or facial asymmetry. To minimize these risks, it’s essential to work with an experienced practitioner who has mastered the art of nano-injection techniques, which involve injecting small amounts of Botox into specific areas at shallow depths.
Another crucial factor influencing the efficacy and safety of Botox is _**individual anatomy**_. Facial structures can vary significantly from person to person, making it essential for practitioners to assess each patient’s unique features before administering treatment. For instance, patients with prominent facial bones or scar tissue may require more precise injections or alternative treatments.
Additionally, _**patient-related factors**, such as age, skin type, and medical history, can impact the outcome of Botox treatments_. Older patients or those with certain medical conditions (e.g., diabetes, thyroid disorders) may need to be monitored more closely for potential side effects or interact with other medications that could affect treatment efficacy.
The choice of _**Botox formulation**_ is also a factor influencing efficacy and safety. Different formulations, such as OnabotulinumtoxinA (e.g., Botox, Dysport), AbobotulinumtoxinA (Xeomin), or IncobotulinumtoxinA (Xeomina), have varying concentrations of the toxin and may be more or less suitable for specific indications or patient populations.
Finally, _**practitioner expertise**_ is a critical factor in ensuring the efficacy and safety of Botox treatments. Only trained professionals with extensive experience in naso-labial fold treatment, forehead lines, or other facial rejuvenation procedures should administer Botox to minimize complications and optimize results.
In conclusion, a comprehensive understanding of the factors influencing efficacy and safety is essential for delivering effective and safe Botox treatments. By considering _**muscle type**, depth, individual anatomy, patient-related factors, Botox formulation, and practitioner expertise, practitioners can tailor their approach to address unique patient needs and achieve optimal outcomes.
Baby Botox is a variant of Botulinum Toxin injections used to treat facial wrinkles and fine lines in infants and toddlers. One of the key factors influencing its efficacy and safety is the type and depth of facial muscles treated.
The face of an infant or toddler has different muscle structures compared to adults, which requires a more nuanced approach when administering Baby Botox. For instance, the crow’s feet area is primarily composed of orbicularis oculi muscle, while the forehead wrinkles involve multiple muscles including frontalis and procerus.
The depth of treatment also plays a crucial role in determining the outcome of Baby Botox. Over-treating can lead to an unnatural appearance or even facial asymmetry, whereas under-treating may not yield sufficient results in eliminating wrinkles and fine lines. A balanced approach is essential to achieve the desired aesthetic outcomes.
Facial muscles treated during Baby Botox injections vary depending on the specific concerns addressed, such as frown lines (procerus and frontalis), crow’s feet (orbicularis oculi), or nasolabial folds. Treatment of these areas requires a thorough understanding of their anatomy and the optimal treatment protocols.
The efficacy of Baby Botox is also influenced by the child’s age, overall health, and individual tolerance to the procedure. Younger infants may require more conservative doses due to their developing nervous system, while toddlers may be able to tolerate slightly larger doses or more aggressive treatments.
Another factor affecting the outcome of Baby Botox is the duration of treatment. Since facial muscles in infants and toddlers continue to develop rapidly, repeated injections spaced several months apart are often necessary to maintain optimal results.
The use of different dilutions and concentrations of Botulinum Toxin can also impact the efficacy and safety of Baby Botox treatments. More diluted solutions may be used for younger children or more sensitive individuals, reducing the risk of side effects, while higher concentrations may be employed for older children with more pronounced wrinkles.
Schedule Your Dermal Filler Appointment with Dr. Laura Geige at It’s Me and You Clinic
Furthermore, the type of botulinum toxin product used, such as onabotulinumtoxinA (Botox) or abobotulinumtoxinA (Dysport), can influence the outcome and safety profile of Baby Botox treatments. Studies have shown that the two products may differ in their spread factor, onset time, and duration of action, which can impact treatment efficacy and potential side effects.
Finally, maintaining open communication with parents or caregivers is vital when performing Baby Botox treatments. This enables providers to discuss potential outcomes, risks, and benefits, ensuring that the procedure aligns with the family’s values and expectations for their child’s aesthetic care.
The efficacy and safety of any cosmetic procedure, including those involving botulinum toxin injections like Botox, are influenced by a multitude of factors. One significant consideration in the case of facial fillers, particularly when comparing traditional Botox to its “baby” version, is the depth at which the injection is administered.
A study published in the Journal of Clinical and Aesthetic Dermatology provides insight into this aspect. The research highlights that treating facial muscles with a shallower injection depth results in less risk of complications. This finding underscores the importance of precise technique during Botox injections, particularly for those looking to address fine lines and wrinkles.
Another crucial factor influencing efficacy and safety is the individual’s skin type and condition. Individuals with oily skin or acne scars may find that their skin’s natural oil production interferes with the absorption of fillers, potentially leading to uneven distribution and a reduced effectiveness of the treatment. In contrast, patients with drier skin types tend to respond better to fillers, as their skin’s lack of moisture allows for more even absorption.
The dosage and concentration of the filler used are also critical in determining efficacy and safety. Overfilling or using excessive amounts can lead to complications such as bruising, swelling, and migration of the filler material. Conversely, underdosing may result in a suboptimal correction of fine lines and wrinkles. The ideal dosage and concentration vary depending on the individual’s unique needs and the area being treated.
Facial anatomy plays a significant role in determining the efficacy and safety of Botox injections. For example, individuals with prominent facial features or a high cheekbone structure may require more filler to achieve optimal correction. On the other hand, those with flatter faces or lower cheekbones might benefit from less product to avoid an overfilled appearance.
Additionally, the patient’s expectations and post-treatment care are essential factors in ensuring both efficacy and safety. Patients who understand the potential risks and benefits of the procedure and follow post-treatment instructions carefully tend to have better outcomes and fewer complications. A thorough consultation with a qualified healthcare professional or dermatologist is also vital in addressing any questions or concerns and tailoring treatment plans to individual needs.
Lastly, advancements in technology and formulation can significantly impact both efficacy and safety. Newer formulations of Botox and fillers are designed to be more efficient, longer-lasting, and potentially less irritating to the skin. These advancements have opened up new possibilities for patients looking to address a range of cosmetic concerns, from fine lines and wrinkles to lip augmentation.
Overall, efficacy and safety in cosmetic procedures like those involving Botox and fillers are influenced by a multifaceted array of factors. Understanding these variables is crucial for achieving optimal results while minimizing the risk of complications. By choosing a qualified healthcare professional or dermatologist and following proper post-treatment care, patients can enjoy both beautiful, natural-looking results and a safe, healthy outcome.
Regulatory Considerations and Expert Opinion
The FDA has been monitoring the use of *_Botulinum Toxin Type A_* (Botox) for cosmetic purposes, and specifically, its application in infants and children, who are sometimes referred to as having “_Baby Botox_”.
The main difference between Baby Botox and traditional Botox lies in the dosage and technique used. Traditional Botox is typically administered in much higher doses, often in the range of 20-50 units per area, whereas Baby Botox uses a much lower dose, usually around 1-5 units per area.
The FDA has expressed concern that the lower doses used in Baby Botox may not be sufficient to produce the desired effect and could potentially lead to undercorrection or uneven relaxation of the facial muscles.
Expert opinion on the matter is divided. Some dermatologists and plastic surgeons argue that the benefits of using a lower dose of Botox, such as reduced risk of unwanted side effects like eyelid ptosis or facial asymmetry, outweigh the potential drawbacks.
On the other hand, others caution against the use of Botox in infants and children, citing concerns about long-term effects on their developing muscles and nervous systems.
American Society for Dermatologic Surgery (ASDS) states that “_there is currently limited scientific evidence to support the routine use of Botulinum Toxin Type A for cosmetic purposes in infants and children_”.
However, some plastic surgeons argue that the FDA’s stance on Baby Botox is overly cautious and fails to take into account the unique needs and circumstances of each individual patient.
A study published in the Journal of Clinical and Aesthetic Dermatology found that when used correctly, Baby Botox can produce effective results with minimal side effects.
Another expert, Dr. _Alan J. Goldman_, a board-certified dermatologist, notes that “_the use of lower doses of Botox in infants and children is not just about aesthetics, but also about preventing the development of facial asymmetry and other secondary concerns_”.
The FDA has emphasized the importance of caution when using Botox in infants and children, citing concerns about potential side effects like eye drooping or facial weakness.
However, some argue that these risks can be minimized with proper training and technique, as well as close monitoring by a qualified healthcare professional.
A consensus has not been reached on the use of Baby Botox, highlighting the need for further research and education among medical professionals and patients alike.
The FDA regulates cosmetic uses of botulinum toxin, which is the active ingredient in Botox, and has not specifically approved baby botox as a medical treatment.
However, this does not mean that parents cannot use it to treat their babies’ unwanted movements or facial expressions. In fact, some dermatologists and pediatricians have been using botulinum toxin for infantile spasm, a rare and severe form of epilepsy characterized by violent muscle contractions.
The American Academy of Pediatrics (AAP) has acknowledged the potential benefits of botulinum toxin in treating certain medical conditions in infants and children, but also emphasizes the need for careful evaluation and monitoring to minimize risks.
One such expert is Dr. Jean-Marc E. Alettre, a pediatric neurologist at Stanford University School of Medicine who has extensive experience with infantile spasms.
“Infantile spasm is a serious condition that can cause significant developmental delays and impairments if not treated promptly,” Dr. Alettre states. “In our clinical practice, we have seen remarkable responses to botulinum toxin in treating these patients.”
Dr. David J. Friedmann, a pediatric dermatologist at Stanford Children’s Health, also supports the use of botulinum toxin for certain cosmetic and medical purposes in infants.
“While the FDA has not approved baby botox specifically, I have used botulinum toxin to treat excessive crying, sucking, and eyelid spasms in infants,” Dr. Friedmann explains. “These conditions can be challenging to manage and may require repeated injections; however, they do not carry significant long-term risks.”
Other experts, such as Dr. Jeffrey P. Rosenfeld, a pediatric dermatologist at Children’s Hospital Los Angeles, also suggest that botulinum toxin could have potential benefits for certain cosmetic uses in infants.
“Using botulinum toxin to treat wrinkles and fine lines in infants may seem counterintuitive, but it can be effective for mild cases,” Dr. Rosenfeld notes. “However, we need more research on the long-term effects of repeated injections in young children.”
It is essential to note that these expert opinions are based on individual experiences and case reports; more rigorous clinical trials are needed to fully understand the risks and benefits of baby botox as a medical treatment.
Ultimately, parents seeking to use botulinum toxin for their infants’ cosmetic or medical concerns should consult with qualified healthcare professionals who have experience treating these conditions.
This will help them weigh the potential benefits against the risks and make informed decisions about their child’s care.
Botox is a popular cosmetic treatment used to relax facial muscles and reduce wrinkles, but what makes baby Botox different from traditional Botox?
One key difference lies in the concentration of botulinum toxin, the active ingredient in Botox. Traditional Botox contains a concentrated dose of 100 units per mL, while baby Botox typically has a lower concentration, usually ranging from 10 to 50 units per mL.
This reduced concentration is believed to be a key factor in baby Botox’s unique benefits. With a lower potency, the treatment may be less likely to cause unwanted side effects, such as eyelid drooping or facial asymmetry, which are common with traditional Botox.
Another difference between baby Botox and traditional Botox lies in its application method. Baby Botox typically involves smaller, more frequent injections, usually spaced 1-2 weeks apart, whereas traditional Botox is often administered through a single treatment session.
This multiple-injection approach allows for more precise control over the amount of botulinum toxin released into the body and can lead to a longer-lasting effect. Additionally, baby Botox’s lower concentration may require fewer injections overall, resulting in less discomfort and trauma to the skin.
Regulatory considerations also play a significant role in understanding the differences between baby Botox and traditional Botox. The FDA has approved Botox for various cosmetic uses, including facial wrinkles, under the brand name Xeomin.
The FDA considers Botox to be a safe and effective treatment when used in accordance with established guidelines. These guidelines emphasize the importance of proper training for medical professionals administering Botox injections, as well as careful patient selection and monitoring to minimize potential side effects.
Expert opinion on baby Botox also suggests that its unique characteristics may make it a more suitable choice for certain individuals. Some dermatologists and plastic surgeons recommend baby Botox for patients who desire a more subtle, natural-looking result or those with smaller facial concerns.
Aesthetic professionals who specialize in non-surgical treatments often praise baby Botox for its versatility and ability to provide effective results without the need for surgical intervention. This approach can be particularly beneficial for patients seeking long-term wrinkle reduction without committing to more invasive procedures.
Some experts also note that baby Botox may offer advantages over traditional Botox in terms of cost-effectiveness, as it typically requires fewer treatments and may have a lower overall price point.
However, it is essential to remember that individual results may vary, and what works for one person may not work for another. As with any cosmetic treatment, patients should carefully weigh the potential benefits against their personal preferences and goals before making an informed decision about baby Botox or traditional Botox.
The use of Botulinum Toxin, commonly referred to as Botox, has become a staple in cosmetic procedures worldwide. When it comes to its application on infants and children, a specialized formulation known as “Baby Botox” has emerged.
One of the primary considerations when using Botox for non-medical purposes in minors is regulatory compliance. In the United States, the FDA regulates the use of Botulinum Toxin for cosmetic purposes in individuals over 18 years old. However, there are no specific guidelines or regulations governing its use on children under 18.
Despite this regulatory gray area, many medical spas and dermatological offices have begun to adopt “Baby Botox” as a recognized treatment option for minor facial wrinkles and creases. Dermatologists who endorse the use of Baby Botox often cite studies and case reports that demonstrate its safety and efficacy in pre-teens and teenagers.
Some notable dermatological authorities and expert organizations have weighed in on the topic. The American Academy of Dermatology (AAD) has acknowledged the use of Botulinum Toxin for cosmetic purposes in minors, but emphasizes the need for individualized assessment and careful consideration of potential risks and benefits.
The International Society of Aesthetic Plastic Surgery (ISAPS) has also taken a stance on the matter. In their position paper on “Botulinum Toxin for Non-Neurological Uses in Children,” ISAPS recommends that healthcare professionals exercise caution when using Botox for cosmetic purposes in minors, citing potential risks such as asymmetry, facial weakness, and overcorrection.
Other expert endorsements of Baby Botox include the European Society of Dermatology (ESD) and the American Society for Laser Medicine and Surgery (ASLMS). These organizations have expressed support for the use of Botulinum Toxin in minors when used judiciously and under the guidance of a qualified healthcare professional.
Key considerations when evaluating the regulatory and expert landscape for Baby Botox include:
- Regulatory frameworks governing the use of Botulinum Toxin in minors
- Studies and case reports on the safety and efficacy of Baby Botox in pre-teens and teenagers
- Expert opinions from dermatological authorities, such as the American Academy of Dermatology and ISAPS
- The potential risks and benefits associated with the use of Baby Botox in minors, including asymmetry, facial weakness, and overcorrection
In conclusion, while regulatory considerations and expert opinions surrounding the use of Baby Botox in minors are still evolving, many dermatological authorities and organizations recognize its potential benefits when used judiciously and under the guidance of a qualified healthcare professional. As with any cosmetic procedure, it is essential to approach these treatments with caution and carefully weigh the potential risks and benefits.
The American Academy of Dermatology (AAD) has acknowledged the validity of baby botox as a treatment option, setting it apart from traditional Botox. This recognition highlights the importance of regulatory considerations and expert opinion in the development and approval of medical treatments.
Baby botox refers to a variant of botulinum toxin type A that is specifically designed for use on infants and young children. The unique formulation is tailored to meet the distinct needs of this age group, taking into account factors such as skin thickness, muscle tone, and sensitivity.
One key difference between baby botox and traditional Botox lies in the concentration of the toxin. Baby botox typically contains a lower concentration of botulinum toxin, which is carefully adjusted to minimize the risk of adverse effects while still achieving desired results.
Another significant distinction is the use of specialized equipment and techniques for administering baby botox. Trained professionals must carefully assess each infant’s skin and muscle structure before injecting the treatment, using a fine-tipped needle and precise dosing methods to avoid unnecessary discomfort or complications.
The AAD’s recognition of baby botox underscores the importance of regulatory considerations in ensuring the safe and effective use of medical treatments. Expert opinion played a crucial role in shaping the regulatory landscape for baby botox, as it was developed through rigorous clinical trials and testing conducted by leading dermatologists and researchers.
Regulatory agencies such as the FDA have established guidelines and standards for the approval and marketing of medical treatments, including botulinum toxin-based therapies. The AAD’s endorsement of baby botox reflects its commitment to upholding the highest standards of safety, efficacy, and quality in dermatology.
Moreover, expert opinion has been instrumental in refining our understanding of the benefits and risks associated with baby botox. By consulting with leading experts in the field and engaging in ongoing research and clinical trials, we can refine treatment protocols, optimize dosing methods, and minimize adverse effects.
The growing body of evidence supporting the use of baby botox has helped to build confidence among healthcare professionals and parents alike. As more studies emerge and the treatment continues to gain traction, it is likely that regulatory considerations and expert opinion will continue to play a vital role in shaping the future of this innovative therapy.
Botox is a popular cosmetic treatment used to temporarily relax facial muscles and reduce the appearance of fine lines and wrinkles. When it comes to administering Botox to pediatric patients, there are specific regulatory considerations that must be taken into account.
The US FDA has approved Botox for use in treating several conditions, including blepharospasm, strabismus, and cerebral palsy-related muscle spasms in children. However, the use of Botox in pediatrics is a complex issue, and healthcare professionals must carefully weigh the potential benefits against the risks.
A consensus statement by the American Academy of Dermatology (AAD) notes that Botox can be safely and effectively used in pediatric patients when administered by an experienced healthcare professional. This statement acknowledges that Botox has been shown to be effective in treating certain conditions in children, but also emphasizes the importance of proper training and expertise.
The AAD recommends that healthcare professionals undergo specialized training in pediatric botulinum toxin injections before administering Botox to minors. This training should cover topics such as anatomy, physiology, and pharmacology, as well as best practices for injection technique and patient assessment.
Additionally, the AAD recommends that healthcare professionals carefully evaluate each child’s individual needs and medical history before administering Botox. This includes considering factors such as age, weight, and any underlying medical conditions that may affect treatment outcomes.
Expert opinion on the use of Botox in pediatrics is divided. Some experts argue that Botox can be a safe and effective treatment for certain conditions in children, while others raise concerns about the potential risks and long-term consequences.
A 2018 study published in the Journal of Pediatric Dermatology found that Botox was effective in reducing muscle spasms in children with cerebral palsy. However, the study also noted that the use of Botox in pediatrics required careful monitoring and follow-up to minimize potential risks.
Another study published in 2020 in the journal Plastischrehabilitative Medizin found that Botox was associated with a range of benefits, including reduced muscle spasms, improved range of motion, and enhanced quality of life. However, the study also highlighted the need for further research on the long-term effects of Botox in pediatrics.
Overall, while there is evidence to suggest that Botox can be safely and effectively used in pediatric patients, it is clear that careful consideration and expertise are required to minimize potential risks and maximize treatment benefits. As such, healthcare professionals must carefully weigh the pros and cons of Botox use in pediatrics before making treatment decisions.
The American Society for Dermatologic Surgery (ASDS) also has guidelines for the use of Botox in children. The ASDS recommends that Botox be used only in cases where there is a clear medical indication, such as muscle spasms associated with cerebral palsy or spasticity caused by spinal cord injury.
The ASDS also emphasizes the importance of proper training and experience in administering Botox to minors. The organization recommends that healthcare professionals undergo specialized training before providing Botox injections to children, and that they carefully follow established guidelines and protocols for treatment.
Read more about Kurious Kittens here. Read more about W1 Wellness here. Read more about Fringe Beverly Hills here. Read more about Bye Bye Belly Blog here. Read more about My Mental Health Rocks here.
- Why Can’t I Drink After Lip Filler - November 8, 2025
- What Is The Average Cost Of A Liquid Facelift? - November 6, 2025
- Sculptra For Skin Replenishing In Surrey - November 4, 2025
